What is Pneumonia?
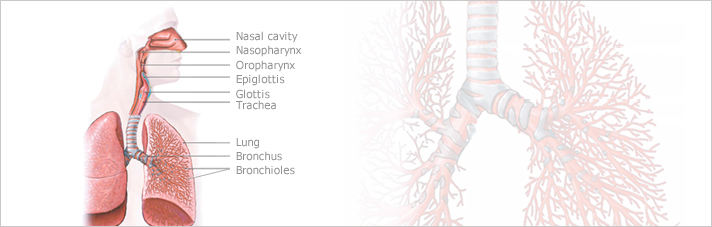
The lungs are a pair of breathing organs located within the chest.
Their function is to remove carbon dioxide from the air and bring oxygen to the blood. Pneumonia is a serious infection that affects the lungs. It is caused by viruses, bacteria, fungi, or the parasites, but most commonly pneumonia is caused by bacteria and viruses.
Community-acquired pneumonia (CAP) is an infection of the lungs that involves the small air sacs (alveoli) and tissues that surround them. CAP is a bacterial infection that is acquired in the community setting while other forms of pneumonia such as hospital-acquired pneumonia can be acquired while a patient is in a hospital or nursing home setting.
What are the risk factors of Pneumonia?
Factors associated with an increased risk of pneumonia include:
- Age. If you are age 65 or older, particularly if you have other conditions that make you more prone to developing pneumonia, you are at increased risk of pneumonia. Very young children, whose immune systems are not fully developed, also are at increased risk of pneumonia.
- Certain diseases. These include immune deficiency diseases such as HIV/AIDS and chronic illnesses such as cardiovascular disease,chronic obstructive pulmonary disease (COPD) and other lung diseases, and diabetes. You are also at increased risk if your immune system has been impaired by chemotherapy or long-term use of immunosuppressant drugs.
- Smoking, alcohol abuse.
- Hospitalization in an intensive care unit. People who need mechanical ventilation are particularly at risk because the breathing tube bypasses the normal defenses of the upper respiratory tract, prevents coughing, may allow the stomach's contents to back up into the esophagus* where they can be inhaled (aspirated), and can harbor bacteria and other harmful organisms.
- Having COPD and using inhaled corticosteroids for more than 24 weeks.
- Exposure to certain chemicals or pollutants. Exposure to air pollution or toxic fumes can also contribute to lung inflammation, which makes it harder for the lungs to clear themselves.
- Surgery or traumatic injury. People who have surgery or who are immobilized from a traumatic injury have a higher risk of pneumonia because surgery or serious injuries may make coughing — which helps clear your lungs — more difficult, and lying flat can allow mucus to collect in your lungs, providing a breeding ground for bacteria.
*The esophagus is the tube that carries food, liquids and saliva from your mouth to the stomach.