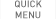What are the Adverse Events associated with FACTIVE?
FACTIVE tablets has been used in over 5 million patients worldwide. Comprehensive safety data collected from >9000 patients treated with FACTIVE in clinical trials and >15,000 patients treated with FACTIVE in postmarketing and phase IV studies demonstrate that FACTIVE is generally well tolerated with a similar frequency of side effects to its comparator antibiotics. The majority of adverse reactions experienced by patients were considered to be of mild to moderate severity.
The overall incidence of adverse events leading to withdrawal in FACTIVE treatment group was equal to or lower than the incidence its comparators treatment group, 3.9% (264/6775) vs. 4.3% (226/5284), respectively.
[Drug-related Adverse Events]
: Possibly or probably related with a frequency of ≥ 1%
Post-Marketing Adverse Reactions
The majority of the post-marketing adverse events reported with FACTIVE were cutaneous and most of these were rash. Some of these cutaneous adverse events were considered serious. The majority of the rashes observed in women and in patients under 40 years of age. In clinical studies, the overall rate of drug-related rash was 2.8% only and 60% of the rashes resolved within 7 days, and 80% resolved within 14 days
The following are additional adverse reactions reported during the post-marketing use of FACTIVE. Since these reactions were reported voluntarily from a population of uncertain size, it is impossible to reliably estimate their frequency or establish a causal relationship to FACTIVE exposure:
- anaphylactic reaction, erythema multiforme, skin exfoliation, facial swelling;
- hemorrhage, increased international normalized ratio (INR), retinal hemorrhage;
- peripheral edema;
- renal failure;
- prolonged QT, supraventricular tachycardia, syncope, transient ischemic attack;
- photosensitivity/phototoxicity reaction
- antibiotic-associated colitis;
- tendon rupture.
Quinolone Class Effect: Cardiac Safety
The quinolones have been associated with ECG changes, particularly prolongation of the QTc interval shown in the following Figure. Such prolongation can lead to the development of potentially fatal cardiac arrythmias.
Because of the occurrence of ECG changes reported with the use of other quinolones, this effect was specifically studied in FACTIVE clinical trials. FACTIVE was found to cause a small, non-significant increase in the QTc interval by a mean of 2.6msec. Considering that the QTc interval lasts, on average, 400msec and that normal variation of the QTc interval in healthy individuals can be up to 75msec, the increase attributable to FACTIVE is small.
[Effects on the QTc Interval]
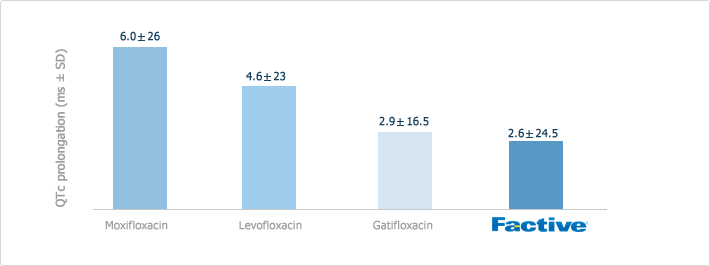
There are no reports of torsades de pointes in FACTIVE-treated patients.
Quinolone Class Effect: Liver Toxicity
The effect of FACTIVE on liver at the recommended dose was devoid of any defined signals for serious toxicity potential. The following table shows the incidence of Alanine Transferase (ALT) elevations on therapy for subjects receiving 320mg FACTIVE.
Most stayed within normal limit (WNL) and comparable rates of elevation were observed with both FACTIVE and pooled comparators. No patients on FACTIVE had elevations greater than 6 X upper limit of normal (ULN).
[Elevated ALT Values on Therapy]
: Patients with Pretreatment Normal ALT Values
| Range | FACTIVE N=3,989 |
All Comparators* N=3,588 |
||
|---|---|---|---|---|
| n | % | n | % | |
| < ULN | 3,800 | 95.3 | 3,443 | 96.0 |
| ULN < 2 X ULN | 162 | 4.1 | 127 | 3.5 |
| 2 to 4 X ULN | 26 | 0.7 | 15 | 0.4 |
| 4 to 6 X ULN | 1 | < 0.1 | 2 | < 0.1 |
| 6 to 8 X ULN | 0 | 0 | ||
| ≥ 8 X ULN | 0 | 1 | < 0.1 | |
* The comparators are consisted of β-lactam antibiotics, macrolides and other fluoroquinolones.
Quinolone Class Effect: Hypoglycemia
[Hypoglycemia with Various Fluoroquinolones]
| Quinolone | Definition of Hypoglycemia |
No. of Patient Evaluated |
Incidence % |
|---|---|---|---|
| FACTIVE | <70 mg/dl | 8,119 | 0 |
| Levofloxacin | <70 mg/dl | 2,386 | 1.6 |
| Moxifloxacin | <50 mg/dl | 2,613 | 0.65 |
*No Cases of Hypoglycemia reported with FACTIVE.