Why does FACTIVE have low propensity to develop resistance?
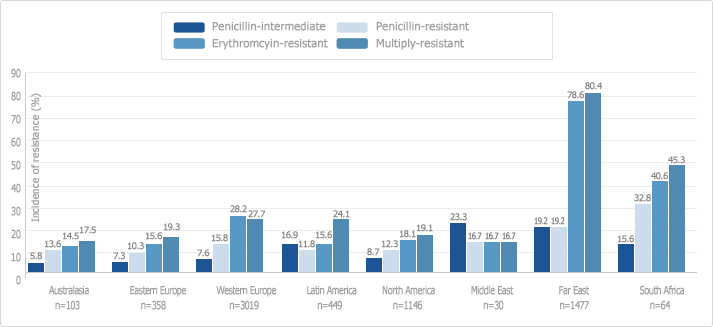
[Worldwide regional differences in the incidence of penicillin-non-susceptibility, erythromycin resistance and multiple resistance phenotypes among isolates of S pneumoniae obtained from elderly patients during PROTEKT (years 1-5)1]
With the increasing prevalence of resistant S Pneumonia, Factive meets an urgent need for powerful first-line therapy with proven clinical efficacy in patients infected with resistant S Pneumonia.
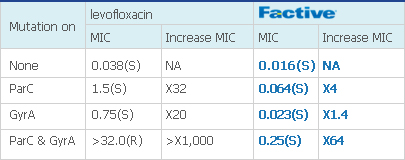
Refer table below. Here’s a strain of pneumococci with a levofloxacin MIC of 0.038, an unusually low MIC for levofloxacin, but a typical gemifloxacin MIC of 0.016. Both MICs are within just one dilution of each other. However, mutations on both targets disproportionately affect levofloxacin, while FACTIVE maintains its potency.
[Table: MIC of levofloxacin & gemifloxacin on Mutated Strains3]
Why is it important to have a dual targeting fluoroquinolone? What does this mean clinically?
Mutations at being found in the lungs of patients with pneumococcal pneumonia. The first step mutations occur at a frequency of approximately 1/107 and second step mutations at 1/105. Therefore, the dual mutations on both targets can be expected to occur at 1/1012. In a patient with pneumococcal pneumonia, there can be 1012 to 1014 organisms in the lung. Therefore, up to a hundred isolates will have both first and second step mutations.4
With marginally active fluoroquinolone, such as levofloxacin, within a few days you could appreciate how a susceptible population of bacteria could be replaced by a resistant population. This is why it is so important to use dual targeting fluoroquinolones such as FACTIVE.
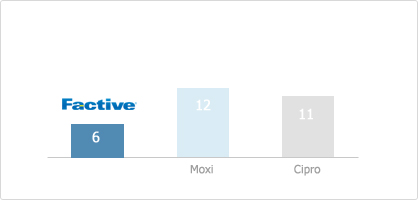
From the study of 50 sequential subcultures of gemifloxacin, ciprofloxacin, gatifloxacin and moxifloxacin to select resistant mutants in pneumococci, FACTIVE induced less number of resistant strains. This shows FACTIVE has low propensity to develop resistance compared to other quinolones.
[Number of Resistant Mutants after Subcultures10]
: Multi-step resistance selection – 50 cycle serial passage
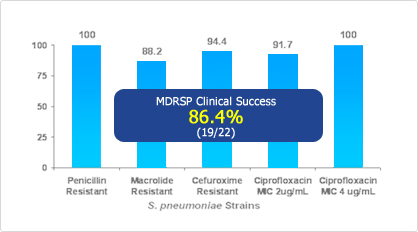
FACTIVE is the first FDA-approved antibiotic to treat MDRSP and is the most potent quinolone in vitro. FACTIVE demonstrated clinical success in 86.4% of patients infected with MDRSP.
[Clinical & Bacteriological Response at Follow Up (%)7,8]