Clinical Efficacy of FACTIVE: Acute Exacerbations of Chronic Bronchitis (AECB)
FACTIVE is a powerful new quinolone indicated for the treatment of acute exacerbations of chronic bronchitis (AECB) caused by a range of the most common causative pathogens. For AECB, FACTIVE has a convenient, short dosing regimen of one 320-mg tablet taken once daily for 5 days.
With broad-spectrum coverage, including S pneumoniae, a short dosing regimen, and proven efficacy, FACTIVE is an excellent new option for the treatment of patients with AECB.
Once-daily, 5-day FACTIVE had excellent clinical success rates in the treatment of AECB as shown in the following study.
[AECB: Clinical Response]
: Excellent Clinical Success with Once-Daily, 5-day FACTIVE vs. 7-day Levofloxacin1
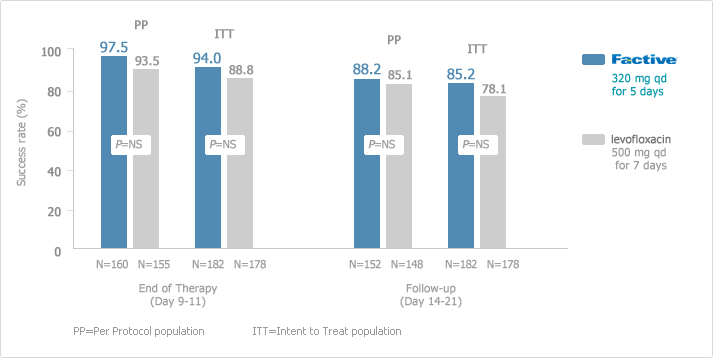
These success rates are particularly important because physicians are increasingly looking for safe and effective alternatives to levofloxacin given the literature reports on levofloxacin-resistant strains of S pneumoniae. This study did not include any isolates of S pneumoniae resistant to levofloxacin. A randomized, double-blind, double-dummy, multicenter, parallel group of patients receiving FACTIVE (n=152) or levofloxacin (n=148). The clinical efficacy of FACTIVE 320 mg once daily for 5 days in AECB was at least as good as levofloxacin 500 mg once daily for 7 days.
[AECB: Clinical Response]
: Excellent Clinical Success with Once-Daily, 5-day FACTIVE vs. 7-day Clarithromycin2
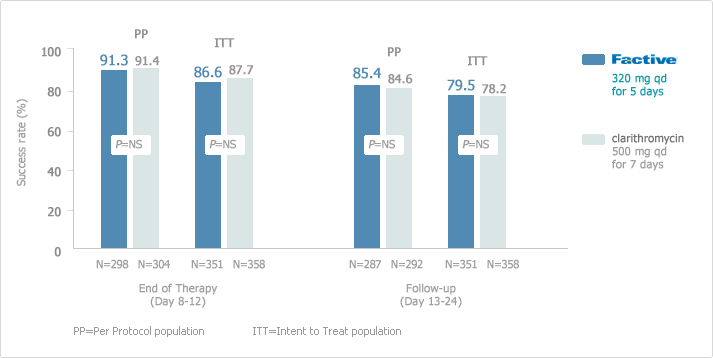
Once-daily, 5-day FACTIVE demonstrated excellent clinical success rates in the treatment of AECB at the end of therapy and follow-up visits compared with clarithromycin in a randomized, double-blind, double-dummy, multicenter, parallel group of patients receiving FACTIVE (n=278) or clarithromycin (n=283). The clinical success rates at follow-up (21-28 days post-therapy) in the clinical per-protocol population (the primary endpoint) were 86.8% (105/121) for gemifloxacin vs. 81.3% (91/112) for ceftriaxone/cefuroxime (treatment difference = 5.5,95% CI 3.9,14.9). The corresponding clinical results in the clinical intention-to-treat (ITT) population were 82.6% (114/138) vs. 72.1% (98/136), respectively (treatment difference = 10.5,95% CI 0.7, 20.4). Thus, FACTIVE had significantly higher clinical success rates than ceftriaxone/cefuroxime. The median time to discharge was 9 days in the gemifloxacin group vs.11 days in the ceftriaxone/cefuroxime group (P = 0.04, Wilcoxon test).
[AECB: Long-Term Clinical Success Rates1,2]
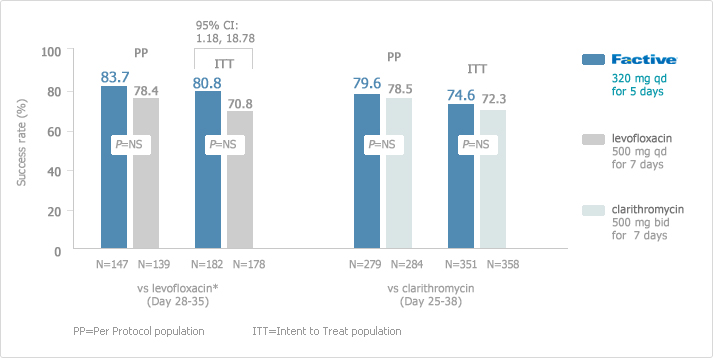
FACTIVE has excellent long-term clinical success rates in the treatment of AECB. Long-term follow-up in the ITT population of Factive was statistically significant compared with levofloxacin (95% CI: 1.18, 18.78).
FACTIVE eradicated H influenzae in 100% of those cultured by the first day of treatment compared to clarithromycin which could not demonstrated complete eradication of H influenzae by day 6.
[AECB: Persistence of H Influenzae in patients1.2]
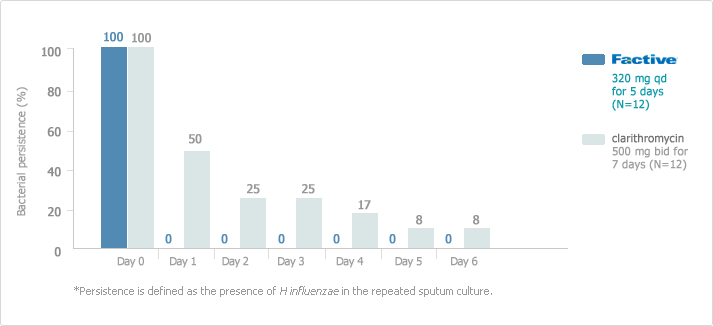
This rapid eradiation is particularly important since longer regimens generally promote resistance.
The clinical relevance of bacterial persistence during the course of therapy has not been established. There was no significant difference in the clinical response between the treatment arms. H influenzae is a key causative pathogen of AECB. Lack of bacterial persistence may be an important component of clinical success. Eliminating bacterial persistence may help patients remain exacerbation-free.