What is the Pharmacokinetics profile of FACTIVE?
FACTIVE is rapidly absorbed after oral administration, achieving its peak plasma concentration around 1 hour after dosing.1 Because it has a relatively long half-life of 7-8 hours,2 it is compatible with a once-daily dosing schedule. There is no accumulation of FACTIVE after multiple dosing over several days.2
Between 25-40% of the oral dose of FACTIVE can be detected unchanged in the urine.3 Dosage adjustments are unlikely to be necessary in patients with mild-to moderate renal or hepatic impairment.
The pharmacokinetics of FACTIVE are not significantly affected by age or by food intake.3
[Pharmacokinetics of FACTIVE3]
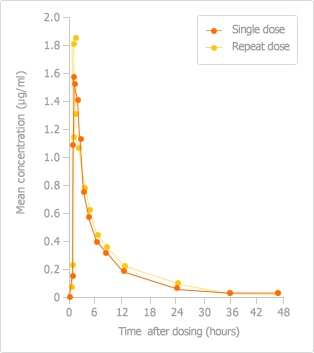
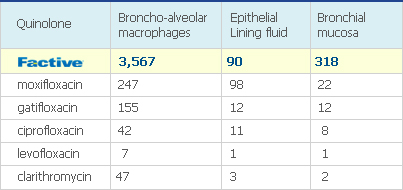
AUC/MIC90 ratios can be used to indicate the likely efficacy of a quinolone antibiotic just as T>MIC can be used to predict the likely efficacy of a β-lactam antibiotic: the higher the AUC/MIC90 ratio, the more effective the quinolone is likely to be. FACTIVE has a superior AUC/MIC90 ratio against streptococci compared with other quinolones.3 As shown the study below, FACTIVE has the highest AUIC, an indicator correlated with clinical outcomes and efficacy.
[24h AUC/MIC90 Ratio on S pneumoniae*†‡4,5,6 ]
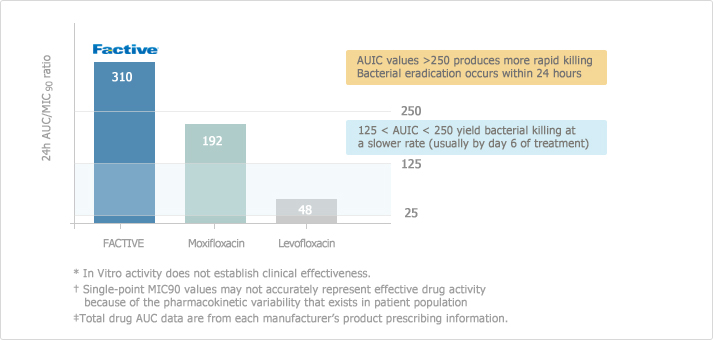
The study shows that FACTIVE distributes in respiratory organ with higher concentration compared to other quinolones.7
[Conc./MIC90 in Respiratory Organ7]
Cost-effectiveness of FACTIVE
Due to shorter dosage regimen, the highest AUIC, and lower reoccurrence rate, some studies proved that FACTIVE is cost-effective. A long-term outcomes in AECB (GLOBE study) and CAP patients support the clinical and financial benefits of using antibiotics with high AUICs. 8,9
[Cost-effectiveness of oral FACTIVE in CAP8]
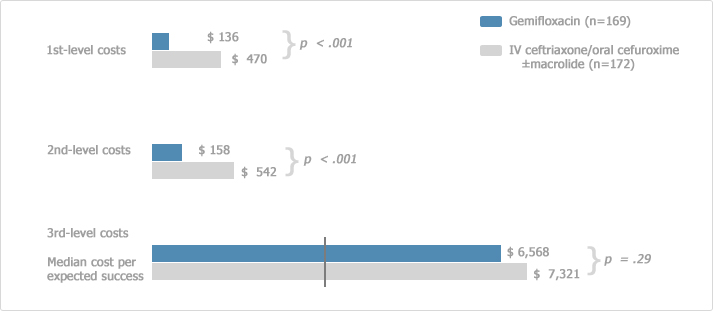
*1st level: antimicrobial acquisition
2nd level: 1st plus preparation, dispensing, and administration costs, and treatment of antimicrobial-related adverse events and clinical failures
3rd level: 1+2st level plus per diem costs for hospital stay related to study drug administration
The cost-effectiveness of oral gemifloxacin was studied by Dr. Ambrose group, compared with IV followed by oral cefuroxime with or without macrolide. And it was observed that Factive has lower median cost per expected success.
[Cost-effectiveness in GLOBE study (AECB)*9]
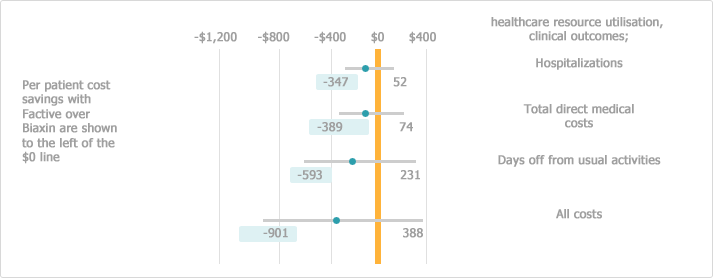
*‘…….the mean direct cost per patient receiving gemifloxacin was $127 less than with clarithromycin.’
*‘…….mean total costs (direct plus indirect) per patient were $329 less for patients receiving gemifloxacin.’
The cost-effectiveness of FACTIVE therapy in AECB was investigated in the Gemifloxacin Long-term Outcomes in GLOBE study and Factive proved to lower mean total treatment cost.